Gadolinium is known to have an association with
Nephrogenic Systemic Fibrosis(NSF). The first case of this was described in
Yale and published in
Lancet. A potential link
between NSF and the application of gadolinium-based contrast agents (GBCAs) was
first described by Grobner
et al in 2006. The risk of gadolinium based contrast agents to trigger NSF
seems to be related to the stability of the agent. Thus, nonionic linear gadolinium
based contrast agents are more likely to trigger NSF than ionic linear agents
both of which are distinctly more likely to trigger the disease than the
macrocyclic agents in patients with reduced renal function. Gadobutrol
(Gadovist, Gadavist) is a second-generation nonionic, multipurpose,
extracellular, macrocyclic gadolinium based contrast agent provided in a 1
molar concentration. As a macrocyclic contrast agent, gadobutrol provides high
chelate stability with substantially less—if any—in vivo release of Gd ions as
opposed to linear gadolinium (old school agents). The release of gadolinium
ions has been linked to an increased risk of NSF in patients with impaired
renal function. The highest prevalence of NSF was associated with Omniscan, Optimark as most of these have a weak binding of gadolinium to the chelate.
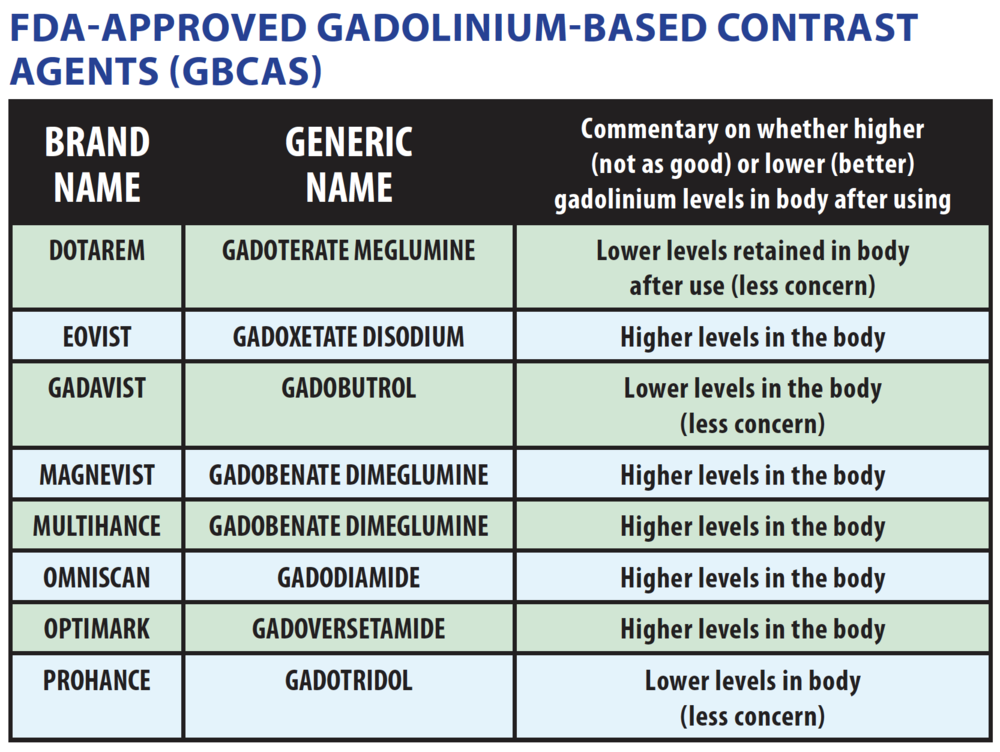
So are these safer? A large study
published in 2017 was a prospective, international, multicenter,
open-label study in 55 centers. Patients with moderate to severe renal
impairment scheduled for any gadobutrol-enhanced MRI were included. All
patients received a single intravenous bolus injection of gadobutrol at a dose
of 0.1 mmol/kg body weight. The primary target variable was the number of
patients who develop NSF within a 2-year follow-up period. A total of 908
patients were enrolled, including 586 with moderate and 284 with severe renal
impairment who are at highest risk for developing NSF. Overall, 184 patients
(20.3%) underwent further contrast-enhanced MRI with other gadolinium-based
contrast agents within the 2-year follow-up. No patient developed symptoms
conclusive of NSF.
Another study by Lauenstein et al,
investigated gadoxetate disodium in 357 patients. No case of NSF was recorded.
Another recent study by
Amet et al investigated the risk of gadoteric acid in 255 patients on
dialysis with no findings of NSF. In addition, Soulez et al reported 2
prospective 2-year studies in 534 patients with either stage 3 chronic kidney
disease (CKD) or stage 4 to 5 CKD. No signs or symptoms of NSF were reported
after administration of gadobenate dimeglumine or gadoteridol. Smorodinsky et al retrospectively
evaluated 1167 patients with chronic liver disease where 72% also had some
degree of renal insufficiency. They did not report any case of NSF.
A recent Canadian
society published their analysis and concluded that “In patients with AKI
and category G4 and G5 CKD (eGFR < 30 mL/min/1.73 m2) and in
dialysis-dependent patients who require Gadolinium based contrast agentss-enhanced
MRI, they can be administered with exceedingly low risk of causing NSF when
using macrocyclic agents and newer linear agents at routine doses.”
Here are images online from medscape education that are very useful on this matter.
A lot of centers in the world are now carrying and using Gadovist
and perhaps we won’t see any NSF?




