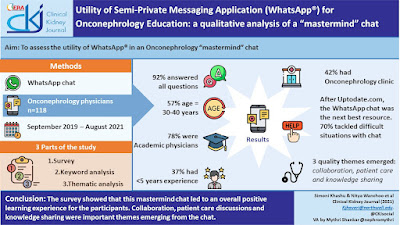
Selective estrogen receptor inhibitors and aromatase inhibitors are the mainstay of therapy for hormonal receptor-positive (HR+) breast cancer; however, most metastatic HR+, human epidermal growth factor receptor 2-negative (HER2-) progress and acquire resistance to endocrine therapies. Cyclin-dependent kinase 4/6 inhibitors (CDK4/6 inhibitors) comprise a new class of drugs that overcome this resistance. Three CDK4/6 inhibitors—palbociclib, ribociclib, and abemaciclib—have been approved for HER2-negative metastatic breast cancers, usually in combination with hormone therapy.


Interestingly, the renal community has seen elevated serum creatinine associated with these agents. Several early trials of palbociclib and ribociclib did not describe the incidence of AKI, whereas clinical trials of abemaciclib have reported that up to 25% of patients experienced a rise in creatinine. In vitro studies of abemaciclib have shown that the drug and its major metabolites inhibit renal transporters like organic cation transporter-2, multidrug and toxin extrusion-1 (MATE-1), and MATE2-K, potentially leading to a reversible rise in creatinine without actually changing GFR. Cases have been described that show this pseudo-AKI.
More recently, biopsy proven cases of acute tubular injury also have been noted- 6 cases with tubular and interstitial damage.
Finally, a search of the FAERs database revealed that, in addition to AKI, metabolic disturbances like hypokalemia, hyponatremia, and hypocalcemia may occur while on CDK4/6 inhibitors. Hyponatremia has been reported with ribociclib and with abemaciclib and grade 2 hypokalemia was reported in 20.8% of patients taking abemaciclib.
In summary, the common renal associations with CDK4/6 inhibitors are
Pseudo AKI, ATI, hyponatremia, hypokalemia and hypocalcemia